Combustion analysis: maximizing a system efficiency and safety
In this Application Note, we will see the basic principles of combustion, in particular the differences between perfect (stoichiometric), good and unsafe combustion.
We will also see the relationship between combustion efficiency, system safety, and maintenance.
Combustion and the significance of unburned fuel
Combustion occurs when oxygen (O2) reacts with the fuel and produces energy in the form of usable heat along with CO2 and H2O. Of course, the energy generated can be utilized for a broad variety of uses, depending on the context in which it is produced.
Optimal combustion occurs when energy from the fuel is harnessed in the best way possible, by limiting heat loss and utilizing the fuel completely. Unfortunately, inefficient and potentially unsafe combustion can occur when a system’s condition is substandard due to normal wear and tear as a result of age, malfunctions, or incomplete combustion (fuel/air mixture). This leads to the release of unused/unburnst fuel in the form of hydrocarbons (CxHy). Their presence indicates a loss of efficiency and potentially serious safety risks.
Why monitor hydrocarbons?
Hydrocarbon emissions are – as we have seen – decisive factors in establishing the quality of combustion and determining if a system is safe or needs immediate interventions, given the existence of perfect, good and unsafe combustion.
Perfect (stoichiometric) combustion
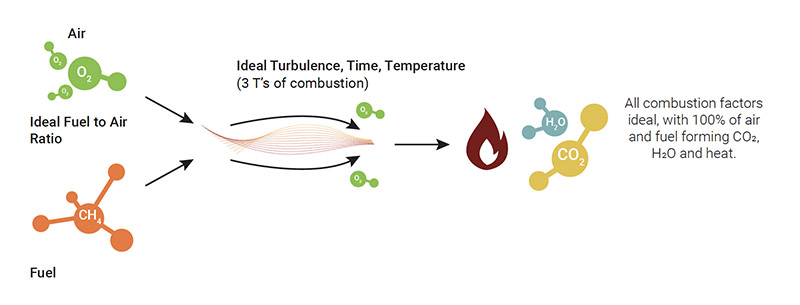
Perfect combustion occurs when the ideal air/fuel ratio is achieved within a system, so as not to produce any waste whilst extracting ALL the energy that the fuel can offer. Even if this type of combustion cannot be realistically obtained, understanding the conditions within which it could occur is useful when striving to improve the performance of a boiler/furnace/heater (or of a burner in general). So, let’s look at the factors that contribute to perfect combustion.
The factors for perfect combustion include:
- An ideal air/fuel ratio, thus the measured and constant supply of air and fuel
- A perfectly-designed burner and combustion process in excellent condition
- Constant characteristics of the fuel
- Ideal turbulence
- Ideal temperature
- Ideal time
- Ideal draft
Assuming these ideal parameters have been achieved, the oxygen and fuel is introduced into a perfectly-functioning burner. As a consequence, the consumption of air and fuel is 100% efficient and CO₂, H₂O and heat is produced without any combustion waste. We understand this is hypothetical and not possible.
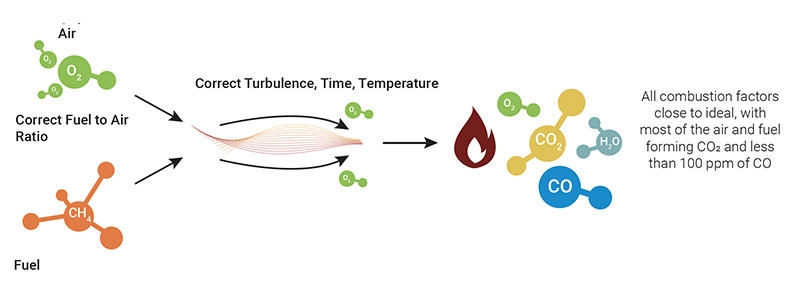
Good combustion
Good combustion occurs when all the factors involved are close to ideal, namely:
- A proper and relatively “good” constant air/fuel ratio
- The burner being kept in good condition, following the manufacturer’s instructions
- The system is properly setup at installation to manufacturer’s specifications
- The characteristics of the fuel are relatively constant
- The turbulence and boiler draft are close to optimal
Under such conditions, the air and fuel combine to form water (H₂O), carbon dioxide (CO₂) and heat, with a CO emission not exceeding 100 ppm. The system uses close to 100% of the fuel with almost zero hydrocarbon emission, thus maximizing the combustion efficiency.
Incomplete combustion
Unsafe or poor combustion occurs when there are drops in efficiency due to wear and tear on the equipment or as a result of malfunctions or improper setup/installations which, as a whole, lead to a loss of energy, unburned fuel or excessive emissions. The first sign of this condition is the emission of hydrocarbons or CO, as an indicator of the inefficiency of the burner and of the need for an immediate inspection and maintenance operations.
Factors of inefficient/incomplete combustion:
- Unstable air/fuel ratio (too fuel rich or too air rich)
- Defective burner
- Relatively inconstant characteristics of the fuel
- Inadequate turbulence and improper mixing of air and fuel
- Draft is not ideal
- Flame impingement
- Inadequate combustion air
- Inadequate venting
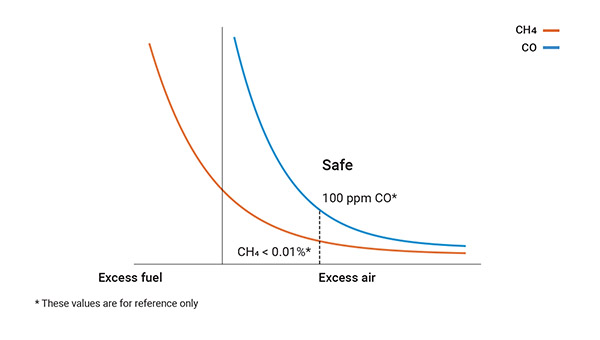
Under such conditions, air and fuel give rise to water (H₂O), carbon dioxide (CO₂) and heat, with the additional production of monoxide (CO) over 100 ppm and a significant quantity of hydrocarbons (CxHy) in the form of unburned fuel.
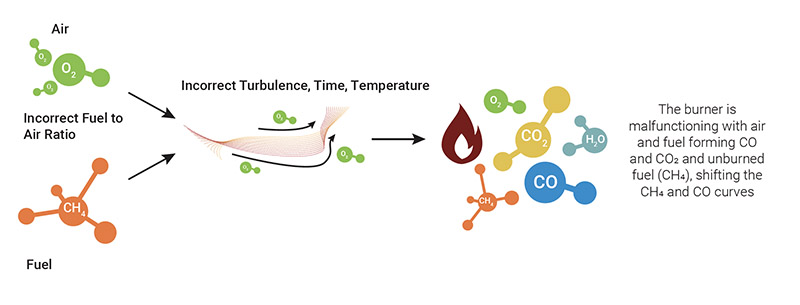
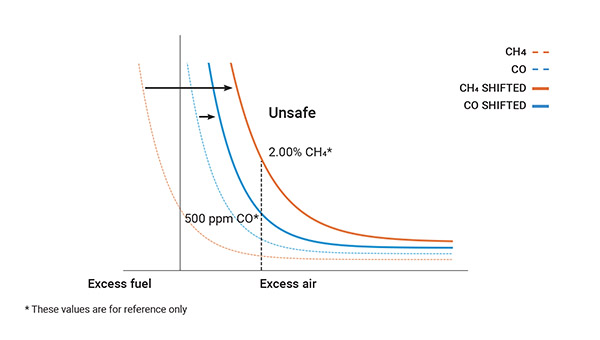
Maximize combustion: monitoring maintenance and safety
Maximum maintenance efficiency is defined as a combustion process being carried out as GOOD combustion within a system operating as designed by the manufacturer (with no defects, damage, etc.).
As is evident, a system’s safety is closely connected to the efficiency of the maintenance. Indeed, old, deteriorated, corroded or broken components lead to an increase in risks and a loss of a system’s performance. To avoid such a situation, it is therefore essential to:
- Carry out the maintenance required by the manufacturer and by laws/standards.
- Carefully monitor parameters associated with combustion, including the amount of excess O₂ necessary for achieving good combustion along with carbon monoxide (CO) and hydrocarbon emissions, draft, temperatures, amongst others.
Precisely because of the importance and cruciality of maintenance, the boiler technician must be equipped with suitable tools for carrying out their tasks quickly and precisely, so as to guarantee system efficiency, safety, productivity for their clients while covering them for potential liability.
Seitron analyzers, such as the Novo, were created precisely to satisfy this need.
Are you interested to know more? Contact us!
Download the full pdf version: Combustion analysis: maximizing a system efficiency and safety