What are fossil fuels and what effects do they have?
Fossil fuels are energy sources formed from the decomposition, in the absence of oxygen, of living matter, subjected to sedimentation, pressure and heat over millions of years.
Coal, oil and natural gas are the three most widely used energy sources on the planet, and the best known fossil fuels. Let’s try to understand what the differences are between the three in terms of emissions and what the risks associated with their use are.
Coal
There are many varieties of coal used in combustion processes around the world, but the most common are anthracite, bituminous and sub-bituminous coal, and lignite. When coal is burned, a considerable amount of carbon dioxide is released, in relation with the percentage of carbon present in the coal. This is also the reason why to burn coal you need more oxygen than other fuels, as we have already seen in the post dedicated to combustion. Ideally for each atom of carbon, you need two of oxygen.
The combustion of coal, precisely because, in reality, it cannot take place by a stoichiometric reaction, also gives rise to other toxic compounds, such as NOx, SO₂ and SO₃.
Oil
Petroleum-based fuels are typically a mixture of heavy hydrocarbons, with hydrogen quantities greater than those found in coal. At the same time, oil contains less carbon than coal, which means that less oxygen is required to achieve complete combustion. It follows that the combustion of oil produces less carbon dioxide than coal, but still more than natural gas.
Natural gas
Natural gas, such as methane for example, requires much less oxygen for complete combustion, precisely because of the greater quantity of hydrogen compared to carbon. If we take methane and analyse its chemical composition, we can easily see why this is so. The molecule is, in fact, made up of one carbon atom and four hydrogen atoms: CH₄.
For this reason then, natural gas is less polluting than either coal or oil. However, it must be borne in mind that, even in this case, if there is combustion with a lack of air, some hydrocarbons will be produced that can lead to safety risks.
However, burning natural gas produces fewer greenhouse gases than other fossil fuels. If we compare it to oil and coal, we have on average an approximate 30 and 45% lower CO₂ production respectively.
The main pollutant produced by the combustion of gas, in addition to CO₂, is NOx, while the emission of SO₂ and particulate matter is negligible.
Risks to health and the environment
As we have seen, the combustion residues of coal, oil and gas are among the main causes of the production of greenhouse gases and other compounds that are hazardous to health and the environment. In particular, we know about the central role of carbon dioxide in the greenhouse effect. So let’s briefly consider the consequences in relation to NOx and SOx.
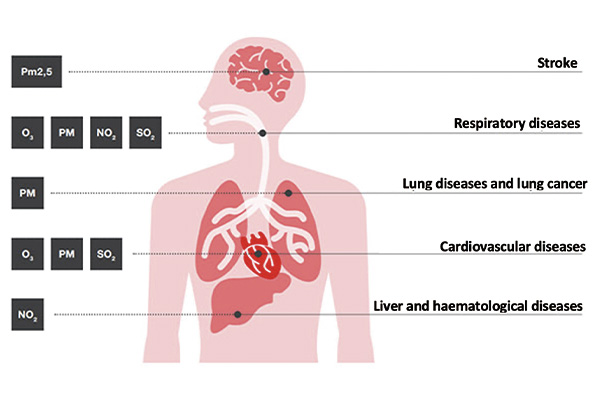
Among nitrogen oxides, having particular toxicological relevance is NO₂, which causes irritation of the distal portion of the respiratory system, with consequences such as asthma, chronic bronchitis, and pulmonary emphysema. NO₂ can also interfere with the gas exchange of plant leaves, causing necrosis and chlorosis.
Nitrogen oxides also contribute to the formation of acid rain, with harmful consequences for aquatic and terrestrial ecosystems.
Among the sulfur oxides, SO₂, as we saw in a previous post, is one of the most aggressive and dangerous air pollutants.
Long exposure to SO₂ can cause serious consequences for the respiratory system, such as tracheitis, pneumonia, and bronchitis. As already mentioned, SO₂ is also responsible for acid rain, with particularly negative consequences for plants and aquatic life. At low concentrations, it leads to a slowdown in the growth of plants, while, at high doses, it can kill them.
Below is a visual summary of the effects of exposure to NO₂ and SO₂, and the risks associated with particulate matter, which, as we have seen, is one of the major pollutants of our cities.